Cannabinoids in medical marijuana can both increase the efficacy of chemotherapy drugs and also minimize the often uncomfortable side effects of conventional cancer treatment, according to a new scientific review of available evidence.
The 23-page paper, published online this month in the journal Pharmacology & Therapeutics, assesses a range of clinical and preclinical findings that “mainly relate to combination treatments for glioblastoma, hematological malignancies and breast cancer, but also for other cancer types.”
“To summarize,” says the report, “the data available to date raise the prospect that cannabinoids may increase the efficacy of chemotherapeutic agents while reducing their side effects.”
It notes that preclinical studies on specific anticancer effects of cannabinoids are limited, looking mostly at whether those compounds are toxic to cancer cells. Other research, authors point out—including into the immune system and cannabinoids' effects on angiogenesis (the formation of new blood vessels), invasion, and cancer metastasis—” is still pending.”
Overall, while the interactions between cannabinoids and chemotherapeutics “constitute a complex subject with many yet unknown variables,” the study says, there are “two important therapy-relevant aspects of the interaction between cannabinoids and chemotherapeutic agents that could potentially benefit cancer patients: firstly, the systemic potentiation of chemotherapeutics by cannabinoids, primarily leading to an extension of life by overcoming therapy resistance and secondly, the reduction of chemotherapy-induced side effects.”
The new paper was authored by a pair of researchers at the Institute of Pharmacology and Toxicology at Rostock University Medical Center, in Germany.
The first portion of the review, focusing on efficacy of chemotherapeutics in combination with cannabinoids, looks in large part at a 2021 Phase 1b clinical study involving a combined THC–CBD oral spray, which showed that patients had a longer survival time when the spray was combined with the drug temozolomide.
It also discusses a variety cannabis of other cancers, including blood and bone marrow cancers, leukemia, breast cancer, skin cancers, bladder and pancreatic cancers, gynecological cancers, colorectal cancers, and numerous others.
The second portion examines cannabinoid therapy as a treatment for chemotherapy side effects, primarily nausea.
“In addition to this well-known antiemetic effect of cannabinoids,” the report adds, “an increasing number of preclinical studies discussed in this review have shown in recent years that cannabinoids can also have a positive effect on other side effects of chemotherapy, such as chemotherapy-induced peripheral neuropathy (CIPN), nephrotoxicity, cardiotoxicity, cystitis, and mucositis.”
While the report deals in less depth with other cancer-related symptoms, it notes that cannabinoids are also administered to relieve cancer-related chronic pain.
“Taken together, the interaction of cannabinoids with currently used chemotherapeutic agents in the context of tumor therapies is of considerable clinical importance, as there are several reasons for the use of cannabinoids in tumor therapies,” it says.
One matter that authors said needs further investigation is the potential for negative interactions between cannabinoids and chemotherapy drugs, noting that there are “several interactions…that are theoretically possible but have not yet been sufficiently investigated” as well as “further findings that give rise to speculation about possible interactions between cannabinoids and chemotherapeutic agents.”
“However,” they added, “it is also possible that cannabinoids trigger yet unknown interactions that benefit the patient.”
Questions also remain around “the extent to which the route of administration influences the interaction with chemotherapeutic agents, particularly in the case of cannabinoids, where the widespread practice of smoking is a major influencing factor.”
“Overall, well-controlled clinical trials are urgently needed for various types of tumors in order to establish cannabinoids as an additional medication against cancer in existing chemotherapies,” the report concludes. “Likewise, the extensive preclinical data available on the interaction of cannabinoids and chemotherapeutic agents at the level of tumor cell death should be extended to include studies on the effects of these combinations at levels of tumor progression, such as angiogenesis, invasion, and metastasis.”
The paper closes with a reminder that while humans have used cannabis for millennia, our ability to scientifically study the plant's effects on the body is only decades old.
“Although cannabinoids have been used in various forms for thousands of years,” it says, “it has only been possible to systematically study their pharmacological mechanisms of action since the discovery of the endocannabinoid system in the early 1990s. Accordingly, they may still hold some as yet undiscovered therapeutically relevant effects on tumor development and progression.”
Separately, U.S.-based researchers last month published what they described as the “largest meta-analysis ever conducted on medical cannabis and its effects on cancer-related symptoms,” finding “overwhelming scientific consensus” on marijuana's therapeutic effects.
The study, published in the journal Frontiers in Oncology, analyzed data from 10,641 peer-reviewed studies—what authors say is more than ten times the number in the next-largest review on the topic. Results “indicate a strong and growing consensus within the scientific community regarding the therapeutic benefits of cannabis,” it says, “particularly in the context of cancer.”
Given what the report calls a “scattered and heterogenous” state of research into the therapeutic potential of marijuana, the authors aimed to “systematically assess the existing literature on medical cannabis, focusing on its therapeutic potential, safety profiles, and role in cancer treatment.”
“We expected controversy. What we found was overwhelming scientific consensus,” lead author Ryan Castle, head of research at Whole Health Oncology Institute, said in a statement. “This is one of the clearest, most dramatic validations of medical cannabis in cancer care that the scientific community has ever seen.”
The meta-analysis “showed that for every one study that showed cannabis was ineffective, there were three that showed it worked,” the Whole Health Oncology Institute said in a press release. “That 3:1 ratio—especially in a field as rigorous as biomedical research—isn't just unusual, it's extraordinary.”
The institute added that the “level of consensus found here rivals or exceeds that for many [Food and Drug Administration]-approved medications.”
Also, last month, government and university researchers from South Korea reported that they had successfully identified a new cannabinoid—cannabielsoxa—produced by the cannabis plant, as well as a number of other compounds “reported for the first time from the flowers of C. sativa.” The team then evaluated 11 isolated compounds in cannabis for antitumor effects in neuroblastoma cells, finding that seven of them “revealed strong inhibitory activity.”
A separate study of medical marijuana patients in Minnesota, published in February, found that people with cancer who used cannabis reported “significant improvements in cancer-related symptoms.” But it also noted that the high cost of marijuana can be burdensome to less financially stable patients and raise “questions about affordability of and access to this therapy.”
The National Cancer Institute (NCI) late last year estimated that between about 20 percent and 40 percent of people being treated for cancer are using cannabis products to manage side effects from the condition and associated treatment.
“The growing popularity of cannabis products among people with cancer has tracked with the increasing number of states that have legalized cannabis for medical use,” the agency said. “But research has lagged on whether and which cannabis products are a safe or effective way to help with cancer-related symptoms and treatment-related side effects.”
The research cited in the NCI post included a series of scientific reports published in the journal JNCI Monographs. That package of 14 articles detailed the results of broad, federally funded cannabis surveys of cancer patients from a dozen agency-designated cancer centers across the country—including in areas where marijuana is legal, permitted only for medical purposes, or still outlawed.
In all, just under a third (32.9 percent) of patients reported using cannabis, with respondents reporting that they used marijuana primarily to treat cancer- and treatment-related symptoms such as difficulty sleeping, pain, and mood changes. The most common perceived benefits were for pain, sleep, stress, and anxiety, and treatment side effects,” the report says.
Separately, another recent study, in the journal Discover Oncology, concluded that a variety of cannabinoids—including delta-9 THC, CBD, and cannabigerol (CBG)—“show promising potential as anticancer agents through various mechanisms,” for example, by limiting the growth and spread of tumors. Authors acknowledged that obstacles to incorporating cannabis into cancer treatment remain, however, such as regulatory barriers and the need to determine optimal dosing.
Other recent research on the possible therapeutic value of lesser-known compounds in cannabis found that a number of minor cannabinoids may have anticancer effects on blood cancer that warrant further study.
While cannabis is widely used to treat certain symptoms of cancer and some side-effects of cancer treatment, there has long been interest in the possible effects of cannabinoids on cancer itself.
A 2019 literature review found that the majority of studies have been based on in vitro experiments, meaning they did not involve human subjects but rather isolated cancer cells from humans, while some of the research used mice. Consistent with the latest findings, that study found cannabis showed potential in slowing the growth of cancer cells and even killing cancer cells in certain cases.
A separate study found that in some cases, different types of cancer cells affecting the same part of the body appeared to respond differently to various cannabis extracts.
A scientific review of CBD last year also touched on “the diverse anticancer properties of cannabinoids” that the authors said present “promising opportunities for future therapeutic interventions in cancer treatment.”
Research from 2023 also found that marijuana use was associated with improved cognition and reduced pain among cancer patients and people receiving chemotherapy
While cannabis produces intoxicating effects, and that initial “high” can temporarily impair cognition, patients who used marijuana products from state-licensed dispensaries over two weeks actually started reporting clearer thinking, the study from the University of Colorado found.
The National Institutes of Health in 2023 awarded researchers $3.2 million to study the effects of using cannabis while receiving immunotherapy for cancer treatment, as well as whether access to marijuana helps reduce health disparities.
On the political side, President Donald Trump's recent choice to serve as the next White House drug czar has called medical marijuana a “fantastic” treatment option for seriously ill patients and said she doesn't have a “problem” with legalization, even if she might not personally agree with the policy.
Under the Trump administration, “marijuana” is also now one of nearly two dozen “controversial or high-profile topics” that staff and researchers at the National Cancer Institute (NCI) are required to clear with higher-ups before writing about.
A leaked agency memo put marijuana and opioids on a list along with vaccines, COVID-19, fluoride, measles, abortion, autism, diversity and gender ideology, and other issues that are believed to be personal priorities of Health and Human Services Secretary Robert F. Kennedy Jr. and President Trump.
NCI is part of the National Institutes of Health (NIH), which itself is part of the Department of Health and Human Services (HHS).
The new memo, first reported by ProPublica, states that NCI staff are required to send the materials to an agency clearance team before publishing on the specified topics.
“Depending on the nature of the information, additional review and clearance by the NCI director, deputy directors, NIH, and HHS may be required,” it advises staff. “In some cases, the material will not need further review, but the NCI Clearance Team will share it with NCI leadership, NIH, and/or HHS for their awareness.”
Written by Ben Adlin for Marijuana Moment | Featured image by Gina Coleman/Weedmaps
The post Medical Marijuana Could Increase Efficacy Of Chemotherapy To Fight Cancer While Reducing Side Effects, Study Suggests appeared first on Weedmaps News.
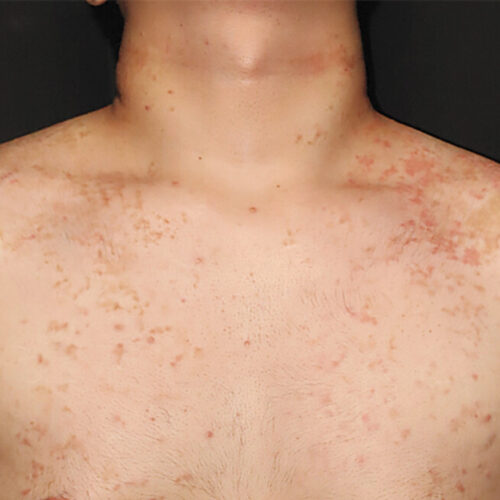
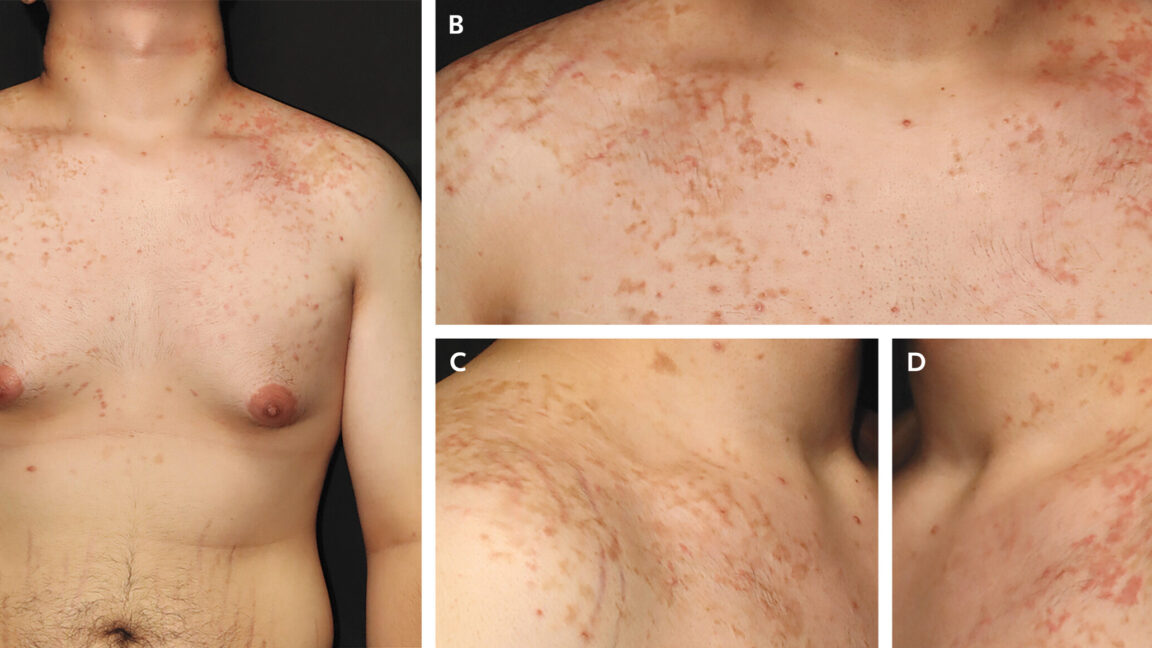
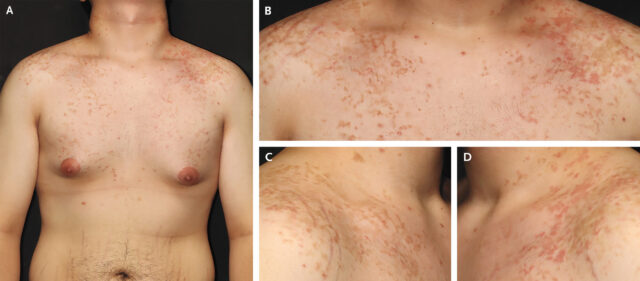 The man's chest and shoulders, showing his rash and hyperpigmentation.
Credit:
New England Journal of Medicine, 2026
The man's chest and shoulders, showing his rash and hyperpigmentation.
Credit:
New England Journal of Medicine, 2026

 Courtesy of Abstrax Tech
Courtesy of Abstrax Tech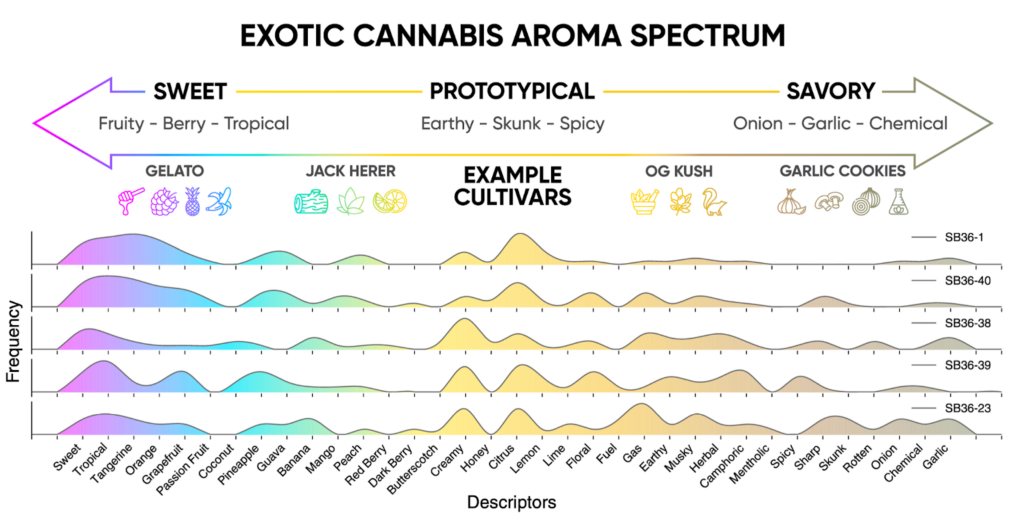 Courtesy of Abstrax Tech
Courtesy of Abstrax Tech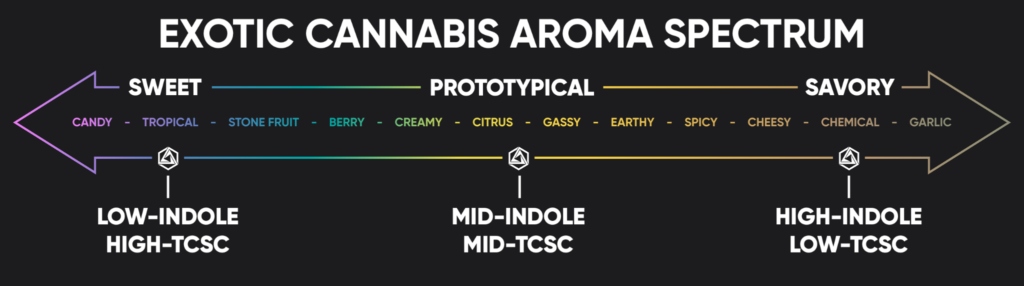 Courtesy of Abstrax Tech
Courtesy of Abstrax Tech