Reading view
RFK Jr. Is Taking on Lyme, but His Whacked-Out Conspiracy Theories Are Worse Than the Disease

New Head of Trump’s Cancer Panel Speculated About Links Between Vaccines and Cancer

The U.S. Might Finally Approve a Sunscreen Ingredient the World’s Been Using for Decades

OpenAI-backed biotech firm Chai Discovery raises $130M Series B at $1.3B valuation
Emerging ‘Super Flu’ Strain Is Spreading Fast. Here’s What You Need to Know

Healing Through Holiday Grief: Finding Peace in Winter Gardening
The holidays can be a very difficult time for those who are grieving a loss. In my own ... Read More
The post Healing Through Holiday Grief: Finding Peace in Winter Gardening appeared first on Garden Therapy.
The Worst Ways RFK Jr. Has Harmed Public Health This Year

Tanning Beds Are Giving Us Skin Cancer Where the Sun Doesn’t Shine

Man shocks doctors with extreme blood pressure, stroke from energy drinks
Sometimes, downing an energy drink can feel like refueling your battery. But with too much, that jolt can turn into a catastrophic surge that fries the wiring and blows a fuse. That was the unfortunate and alarming case for a man in the UK several years ago, according to a case report this week in BMJ Case Reports.
The man, who was in his 50s and otherwise healthy, showed up at a hospital after the entire left side of his body abruptly went numb and he was left with clumsy, uncoordinated muscle movements (ataxia). His blood pressure was astonishingly high, at 254/150 mm Hg. For context, a normal reading is under 120/80, while anything over 180/120 is considered a hypertensive crisis, which is a medical emergency.
The man had suffered a mild stroke, and his extremely high blood pressure was an obvious factor. But why his blood pressure had reached stratospheric heights was far less obvious to his doctors, according to the retrospective case report written by Martha Coyle and Sunil Munshi of Nottingham University Hospital.


© Getty | DANIEL LEAL
Plant-Based Diets Can Work for Kids, but New Study Outlines Concerning Risks

Senator endorses discredited doctor’s book that claims chemical treats autism, cancer
For years, Sen. Ron Johnson has been spreading conspiracy theories and misinformation about COVID-19 and the safety of vaccines.
He’s promoted disproven treatments for COVID-19 and claimed, without evidence, that athletes are “dropping dead on the field” after getting the COVID-19 vaccination. Now the Wisconsin politician is endorsing a book by a discredited doctor promoting an unproven and dangerous treatment for autism and a host of ailments: chlorine dioxide, a chemical used for disinfecting and bleaching.
The book is “The War on Chlorine Dioxide: The Medicine that Could End Medicine” by Dr. Pierre Kory, a critical care specialist who practiced in Wisconsin hospitals before losing his medical certification for statements advocating using an antiparasite medication to treat COVID-19. The action, he’s said, makes him unemployable, even though he still has a license.


© Scott Olson
People Are Already Taking This Unapproved New Weight-Loss Drug

Expanded carrier screening: Is it worth it?
This week I’ve been thinking about babies. Healthy ones. Perfect ones. As you may have read last week, my colleague Antonio Regalado came face to face with a marketing campaign in the New York subway asking people to “have your best baby.”
The company behind that campaign, Nucleus Genomics, says it offers customers a way to select embryos for a range of traits, including height and IQ. It’s an extreme proposition, but it does seem to be growing in popularity—potentially even in the UK, where it’s illegal.
The other end of the screening spectrum is transforming too. Carrier screening, which tests would-be parents for hidden genetic mutations that might affect their children, initially involved testing for specific genes in at-risk populations.
Now, it’s open to almost everyone who can afford it. Companies will offer to test for hundreds of genes to help people make informed decisions when they try to become parents. But expanded carrier screening comes with downsides. And it isn’t for everyone.
That’s what I found earlier this week when I attended the Progress Educational Trust’s annual conference in London.
First, a bit of background. Our cells carry 23 pairs of chromosomes, each with thousands of genes. The same gene—say, one that codes for eye color—can come in different forms, or alleles. If the allele is dominant, you only need one copy to express that trait. That’s the case for the allele responsible for brown eyes.
If the allele is recessive, the trait doesn’t show up unless you have two copies. This is the case with the allele responsible for blue eyes, for example.
Things get more serious when we consider genes that can affect a person’s risk of disease. Having a single recessive disease-causing gene typically won’t cause you any problems. But a genetic disease could show up in children who inherit the same recessive gene from both parents. There’s a 25% chance that two “carriers” will have an affected child. And those cases can come as a shock to the parents, who tend to have no symptoms and no family history of disease.
This can be especially problematic in communities with high rates of those alleles. Consider Tay-Sachs disease—a rare and fatal neurodegenerative disorder caused by a recessive genetic mutation. Around one in 25 members of the Ashkenazi Jewish population is a healthy carrier for Tay-Sachs. Screening would-be parents for those recessive genes can be helpful. Carrier screening efforts in the Jewish community, which have been running since the 1970s, have massively reduced cases of Tay-Sachs.
Expanded carrier screening takes things further. Instead of screening for certain high-risk alleles in at-risk populations, there’s an option to test for a wide array of diseases in prospective parents and egg and sperm donors. The companies offering these screens “started out with 100 genes, and now some of them go up to 2,000,” Sara Levene, genetics counsellor at Guided Genetics, said at the meeting. “It’s becoming a bit of an arms race amongst labs, to be honest.”
There are benefits to expanded carrier screening. In most cases, the results are reassuring. And if something is flagged, prospective parents have options; they can often opt for additional testing to get more information about a particular pregnancy, for example, or choose to use other donor eggs or sperm to get pregnant. But there are also downsides. For a start, the tests can’t entirely rule out the risk of genetic disease.
Earlier this week, the BBC reported news of a sperm donor who had unwittingly passed on to at least 197 children in Europe a genetic mutation that dramatically increased the risk of cancer. Some of those children have already died.
It’s a tragic case. That donor had passed screening checks. The (dominant) mutation appears to have occurred in his testes, affecting around 20% of his sperm. It wouldn’t have shown up in a screen for recessive alleles, or even a blood test.
Even recessive diseases can be influenced by many genes, some of which won’t be included in the screen. And the screens don’t account for other factors that could influence a person’s risk of disease, such as epigenetics, microbiome, or even lifestyle.
“There’s always a 3% to 4% chance [of having] a child with a medical issue regardless of the screening performed,” said Jackson Kirkman-Brown, professor of reproductive biology at the University of Birmingham, at the meeting.
The tests can also cause stress. As soon as a clinician even mentions expanded carrier screening, it adds to the mental load of the patient, said Kirkman-Brown: “We’re saying this is another piece of information you need to worry about.”
People can also feel pressured to undergo expanded carrier screening even when they are ambivalent about it, said Heidi Mertes, a medical ethicist at Ghent University. “Once the technology is there, people feel like if they don’t take this opportunity up, then they are kind of doing something wrong or missing out,” she said.
My takeaway from the presentations was that while expanded carrier screening can be useful, especially for people from populations with known genetic risks, it won’t be for everyone.
I also worry that, as with the genetic tests offered by Nucleus, its availability gives the impression that it is possible to have a “perfect” baby—even if that only means “free from disease.” The truth is that there’s a lot about reproduction that we can’t control.
The decision to undergo expanded carrier screening is a personal choice. But as Mertes noted at the meeting: “Just because you can doesn’t mean you should.”
This article first appeared in The Checkup, MIT Technology Review’s weekly biotech newsletter. To receive it in your inbox every Thursday, and read articles like this first, sign up here.
RFK Jr.’s Health Department Is Pondering a National Men’s Health Initiative

Ars Live Today: 3 former CDC leaders detail impacts of RFK Jr.’s anti-science agenda
The Centers for Disease Control and Prevention is in critical condition. This year, the premier public health agency had its funding brutally cut and staff gutted, its mission sabotaged, and its headquarters riddled with literal bullets. The over 500 rounds fired were meant for its scientists and public health experts, who endured only to be sidelined, ignored, and overruled by Health Secretary Robert F. Kennedy Jr., an anti-vaccine activist hellbent on warping the agency to fit his anti-science agenda.
Then, on August 27, Kennedy fired CDC Director Susan Monarez just weeks after she was confirmed by the Senate. She had refused to blindly approve vaccine recommendations from a panel of vaccine skeptics and contrarians that he had hand-selected. The agency descended into chaos, and Monarez wasn’t the only one to leave the agency that day.
Three top leaders had reached their breaking point and coordinated their resignations upon the dramatic ouster: Drs. Demetre Daskalakis, Debra Houry, and Daniel Jernigan walked out of the agency as their colleagues rallied around them.
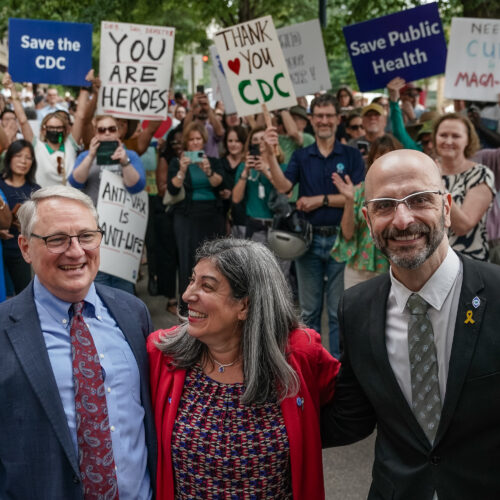

© Getty | Elijah Nouvelage
Joon Care, a Seattle-based mental health startup serving youth, acquired by Handspring Health

Seattle-based mental health startup Joon Care has been acquired by Handspring Health, a New York-based health tech company. Terms of the deal were not disclosed.
“The acquisition is a major step toward building the most clinically rigorous and digitally engaging platform for youth and family mental healthcare in the country,” said Sahil Choudhry, co-founder and CEO of New York-based Handspring, in a LinkedIn post.
Joon launched in 2019 to provide online care for teens and young adults, pairing digital tools with virtual therapy sessions. The company serves patients 13- to 26-years-old who need help with anxiety, depression, disordered eating, sexual and gender identity, academic problems and other challenges. The course of therapy typically runs 16 weeks. The company’s program emphasizes its use of evidence-based care strategies and patient assessments to track progress.
Joon spun out of Seattle’s Pioneer Square Labs (PSL) and raised an initial $3.5 million round in 2020. Two years ago, it announced an additional $6 million investment, which would provide two to three years of operations, CEO Emily Pesce said at the time.
Handspring said in a press release that it would be integrating the companies’ “expert teams,” but did not say if all of Joon’s employees would be retained. The company has roughly 50 employees, based on information on LinkedIn.
GeekWire reached out to Pesce and will update the story if we hear back.
Handspring launched in 2021 and has raised $18.2 million, according to PitchBook. It also provides virtual therapy and online support, serving a slightly larger demographic with patients from 8- to 29-years-old.
Both companies operate multi-state platforms. Joon is licensed to provide care in Washington, Oregon, California, Texas, New York, Delaware and Pennsylvania. Its treatment is covered by 16 insurance companies, according to its website, and includes national giants Aetna and UnitedHealthcare.
Joon also launched a partnership in 2023 with the City of Seattle to provide free care to clients who are referred to the startup through the city’s human services programs. The collaboration appears to be ongoing, and Handspring said it would continue serving families under Joon’s existing contracts with government agencies, as well as treatment covered by insurance companies.
Pesce was a finalist for Startup CEO of the Year at the 2023 GeekWire Awards.
Eli Lilly’s Weight Loss Superdrug Worked So Well During Trials, Some Quit Over Losing Too Much

Step aside, semaglutide—retatrutide is showing the most impressive weight loss results of any obesity drug to date.

Sperm Donor With a Cancer-Causing Gene Fathered at Least 197 Kids

Because only part of the man's sperm carried the mutation, only the second round of genetic testing revealed the problem. By then, it was already too late.

Many States Say They’ll Defy RFK Jr.’s Changes to Hepatitis B Vaccination
